Latest News
Fast MDx receives ISO 13485:2016 certification, setting out the requirements for quality management systems for diagnostics and medical devices.
A review funded by the Bill and Melinda Gates Foundation perfectly explains the greater need for high-throughput Point-of-Care preparedness that only the Fast MDx platform offers.
In a study reported in Nature on 25 September 2023 an immune system biomarker combinations was identified that distinguish Long COVID (LC) cases from those with no persisting symptoms.
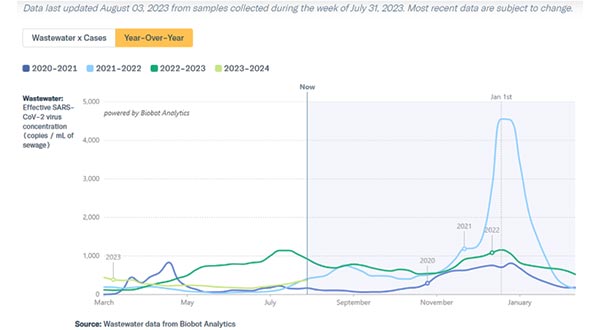
COVID-19 metrics have been on the rise in the U.S. for about a month now, indicating that they’re experiencing a summer surge.
Scotland's national clinical director has said he is concerned about winter amid a summer upswing in Covid cases.
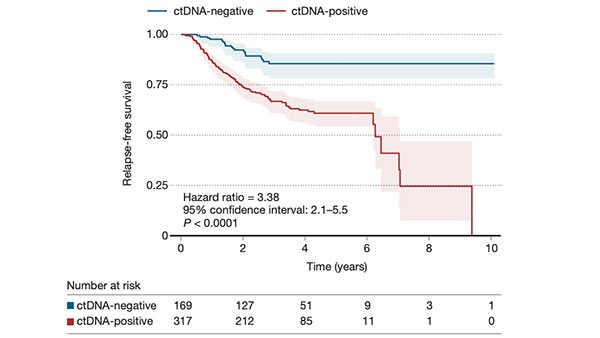
A recent review strongly supports the use of repeated testing for circulating tumour DNA (ctDNA), for prognosis and for monitoring for recurrence of solid tumours.
In the Press/Press Releases
No PR articles found, sorry.
Read the latest information from Fast MDx on LinkedIn.